This inhaler contains medicine capsules and a device body. The capsule is made of special materials, which is easy to puncture, slag-free, and has light weight and less residual powder. (Patent Number: CN202030754273.1)

The Continuous capsule-based dry powder inhaler features multi-capsule storage with sequential dosing functionality.Each press of the button-type piercing mechanism punctures the capsule and automatically loads it into the mixing chamber.

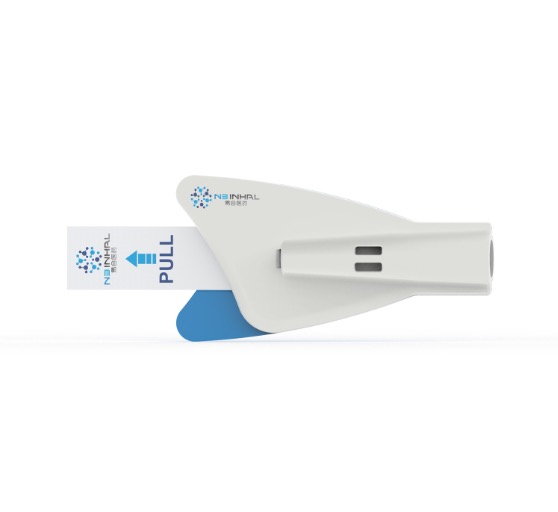
This inhaler contains blister strips and a device body. One dose of medicine is enclosed inside one blister strip. When in use, the users place one blister strip inside the specific place of the device. (Patent number: CN201911132049.1)

This device features a simple structure, portability, ease of operation, intuitive use, low manufacturing cost, and full biodegradability. Users can independently replace the blister strips, enabling multiple uses of the device and reducing per-use costs.
The single-dose disposable dry powder inhaler has a compact design, simple structure and good portability. It is easy and intuitive to operate. The whole device has low manufacturing cost and is biodegradable.

This inhaler contains medicine powder and a device body. The medicine powder that meets the needs of patients is pre-filled inside the device. The specially designed drug-loading unit inside the device can accurately load a single dose for inhalation.

This device consists of a drug capsule and a device body. The device is designed to puncture the capsule safely and easily, leaving no residue. It features an easily replaceable nozzle, offers compatibility with a wide range of formulations, and generates

This inhaler contains medicine disks and a device body. The medicine disc is composed of a plurality of blisters containing drugs which are distributed along the circumference of the disc. (Patent no: CN200780012575.3)

This device contains the formulation for patient use within individual blisters, with a single blister strip containing multiple independent drug-filled blisters. The user slides the device cover downward, causing the internal gears to rotate.





